
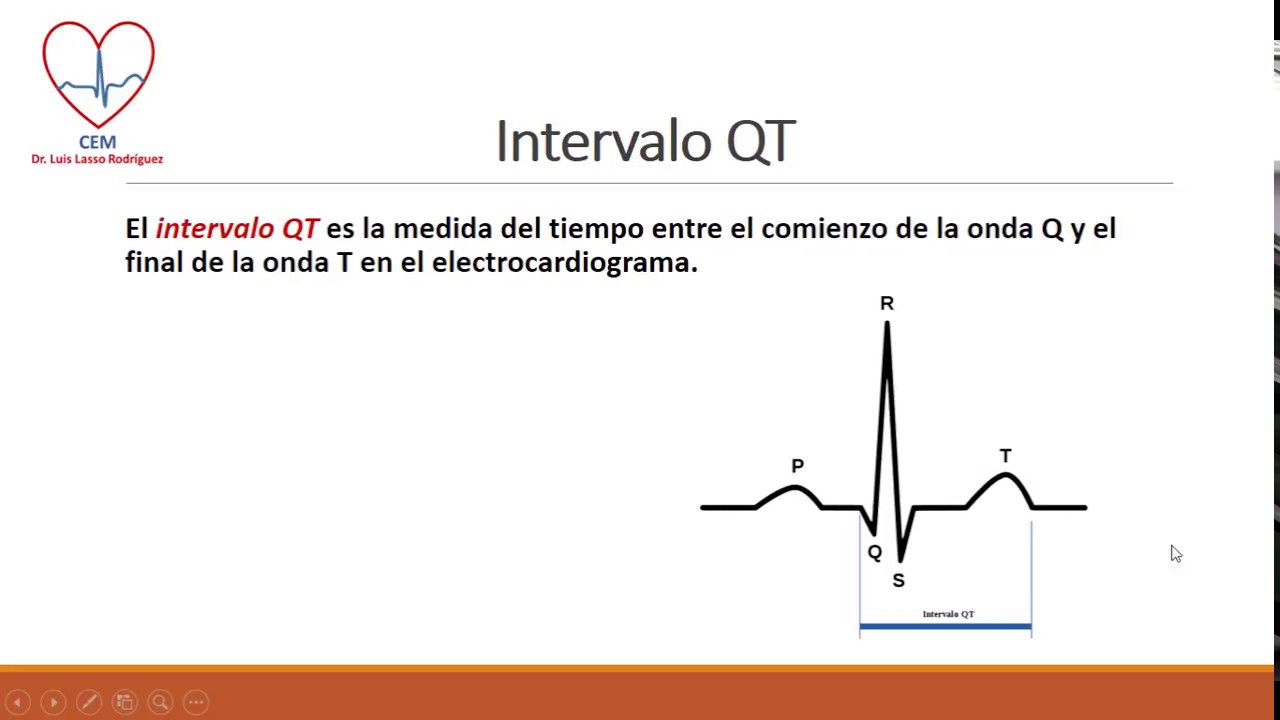
The use of a single 32 mg intravenous dose of ondansetron should be avoided.In addition, Torsade de Pointes, an abnormal, potentially fatal, heart rhythm, has been reported in some patients receiving ondansetron. ECG changes including QT interval prolongation have been observed in patients receiving ondansetron.Report any side effects you experience to the FDA MedWatch program using the information in the "Contact Us" box at the bottom of the page.Īdditional Information for Healthcare Professionals (updated from ).

#Intervalo qt normal ecg professional
#Intervalo qt normal ecg update
It also does not change the recommended lower dose intravenous dosing of ondansetron to prevent post-operative nausea and vomiting.Īs part of the ongoing safety review of ondansetron, FDA continues to assess data about the risk of QT prolongation and will update the public when more information becomes available.
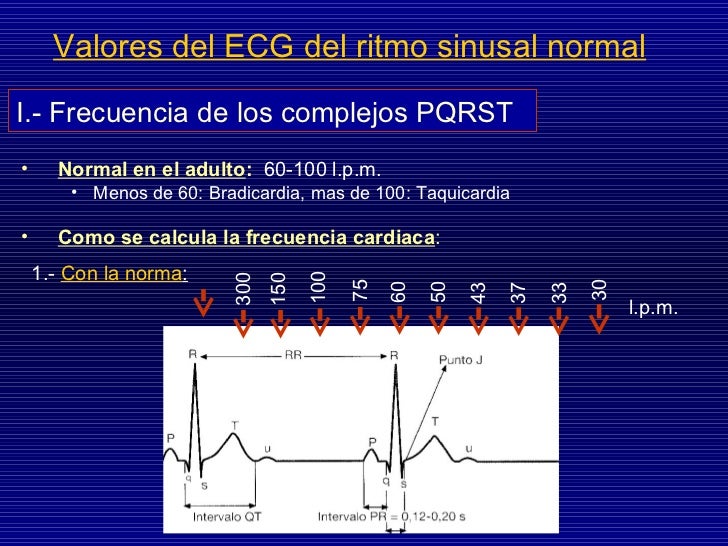
The new information on QT prolongation does not change any of the recommended oral dosing regimens for ondansetron. Information from the new clinical study will be included in the updated drug label.įDA will evaluate the final study results when available, and will work with GSK to explore an alternative single dose regimen that is both safe and effective for the prevention of chemotherapy-induced nausea and vomiting in adults. The updated label will state that ondansetron can continue to be used in adults and children with chemotherapy-induced nausea and vomiting at the lower intravenous dose recommended in the drug label, a dose of 0.15 mg/kg administered every 4 hours for three doses however, no single intravenous dose should exceed 16 mg. GlaxoSmithKline (GSK) has announced changes to the Zofran drug label to remove the 32 mg single intravenous dose. Food and Drug Administration (FDA) is informing healthcare professionals and the public that preliminary results from a recently completed clinical study suggest that a 32 mg single intravenous dose of ondansetron (Zofran, ondansetron hydrochloride, and generics) may affect the electrical activity of the heart (QT interval prolongation), which could pre-dispose patients to develop an abnormal and potentially fatal heart rhythm known as Torsades de Pointes. Īdditional Information for Healthcare Professionals This update is in follow-up to the FDA Drug Safety Communication: Abnormal heart rhythms may be associated with use of Zofran (ondansetron) on.


 0 kommentar(er)
0 kommentar(er)
